Medical - grade precision injection - molded parts are plastic products for medical fields (medical devices, consumables, drug delivery systems). They feature high precision, cleanliness, biocompatibility, and long - term stability. Compared with ordinary parts, their dimensional accuracy error is controlled within ±0.01mm or lower, and the surface roughness Ra is usually ≤0.8μm, meeting the strict adaptability and safety requirements of items like syringe needles and heart stent catheters. Also, as they directly contact human tissues/blood, strict biosafety tests are needed to avoid toxicity and allergenic risks.
Medical - grade precision injection molding requires special electric injection molding machines. These machines have closed - loop servo control systems, controlling injection pressure fluctuation within ±1% and holding pressure repeat accuracy at ±0.5MPa, ensuring product weight difference < 0.1%. For example, KraussMaffei's all - electric machines use linear guides and ball screws, achieving a positioning accuracy of ±0.001mm, avoiding pressure pulsation and oil pollution of traditional hydraulic systems.
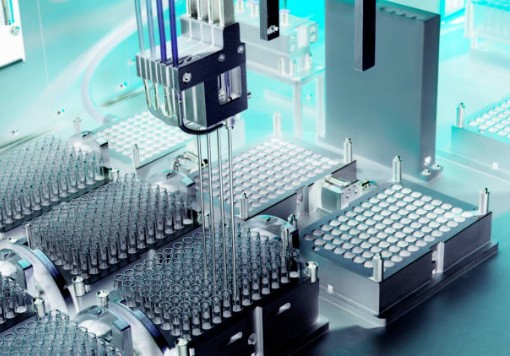
As the core of precision injection molding, molds must have a manufacturing tolerance of ±0.005mm. Their surfaces undergo mirror polishing (Ra≤0.2μm) and are treated with hard chrome plating or diamond - like carbon (DLC) coating to improve wear resistance. Before use, molds pass helium mass spectrometry leak testing for cavity sealing. After every 5,000 moldings, ultrasonic cleaning and precision re - measurement are done to prevent release agent residue and dimensional wear.
Medical - grade injection molding prefers materials meeting ISO 10993, such as PC, PEEK, and PPSU. Take PPSU as an example: it must pass 12 biosafety tests (e.g., cytotoxicity by MTT assay, sensitization by skin patch test, pyrogen by limulus reagent test), and its molecular mass distribution should be narrower than 1.8 for batch stability.
Raw materials should be stored in a constant - temperature (20±2℃) and constant - humidity (≤40%) warehouse. They need to be used within 8 hours after unpacking. Each batch must provide biocompatibility reports, FDA 510(k) certification documents, and undergo incoming inspections for melt index (MI) and color difference (ΔE≤1).

Medical - grade injection molding is carried out in ISO Class 7 (10,000 - class) or higher clean workshops. Some implant - related products require ISO Class 5 (100 - class) laminar flow hoods. The workshop controls airborne particles (≥0.5μm particles ≤3,520 per m3) and settling bacteria (≤10CFU per dish), and ensures surface cleanliness through monthly ATP fluorescence testing.
Operators must wear hooded sterile clothing, masks, and gloves, and enter the workshop after air shower dust removal. All equipment is regularly sterilized with ozone or hydrogen peroxide. Conveyors, hoppers, etc., use stainless steel or food - grade plastic to avoid secondary pollution.
First - piece Inspection: Use a coordinate measuring machine (CMM) for micron - level measurement of key dimensions, with 100% form and position tolerance inspection coverage.
Online Inspection: High - speed vision inspection systems capture 2,000 frames per second to identify defects like weld lines and air holes smaller than 0.1mm2.
Final Inspection: Besides regular physical property tests, accelerated aging tests (e.g., 70℃ for 168 hours) simulate a 5 - year service life. Gamma ray or ethylene oxide sterilization verifies disinfection resistance.
Each injection - molded part is marked with production batches, mold numbers, and operator information via laser or QR code for full - lifecycle traceability. Production data is uploaded to the MES system in real - time, forming electronic files with 150 parameters, meeting FDA 21 CFR Part 11 compliance requirements.
Medical - grade injection - molded parts must comply with multi - national regulations: FDA QSR 820, EU MDR (2017/745), and China's "Medical Device Production Quality Management Specifications (GMP)". Enterprises need ISO 13485 certification and apply for CE, FDA 510(k), or China NMPA registration for specific products to ensure legal market entry.
By strictly following these standards, enterprises can systematically control quality risks, drive high - value and safe product development, and meet the growing global medical market demand.




